Poor, elderly individuals who may qualify for both Medicaid (for being poor) and Medicare (for being elderly, blind, disabled or have ESRD). In these cases, Medicaid serves as a supplemental insurer, covering Medicare coinsurance and deductibles. The generosity of this supplemental coverage for so-called ‘dual-eligibles’ varies across states.
These differences in Medicaid payments arise from two sources of policy variation. First, states differ in their adoption of so-called “lesser-of” policies, which are provisions for Medicaid to pay the lower of (a) Medicare’s cost sharing, or (b) the difference between the Medicaid fee schedule and Medicare’s payment for a service (net of cost sharing).1 Second, Medicaid fee schedules, which vary across states and over time, affect the amount of cost sharing that Medicaid will pay providers in lesser-of states.
In lesser-of states with low Medicaid fee schedules, providers can be paid substantially less when rendering services to duals vs other Medicare beneficiaries, who either pay Medicare’s cost sharing out of pocket or have private supplemental (ie, Medigap) insurance to
cover these expenses.
Unsurprisingly, providers are not as excited to provide care for dual-eligibles when they get paid less money for providing the same services they provide to Medicare beneficiaries (see Mitchell et al 2004, Haber et al. 2014, Zheng et al. 2017).
To measure variation in state Medicaid policies regarding dual eligibles, a paper by Roberts et al. (2020) describes the process of creating a database of these policies. The sources of the database were (i) state Medicaid plans and amendments filed with CMS; (ii) state laws from LexisNexis; (iii) and Medicaid provider manuals, program bulletins, and related online policy documents. Then the authors created a payment index using a nationally representative sample of claims data for evaluation and management services HCPCS codes. The database is publicly available here.
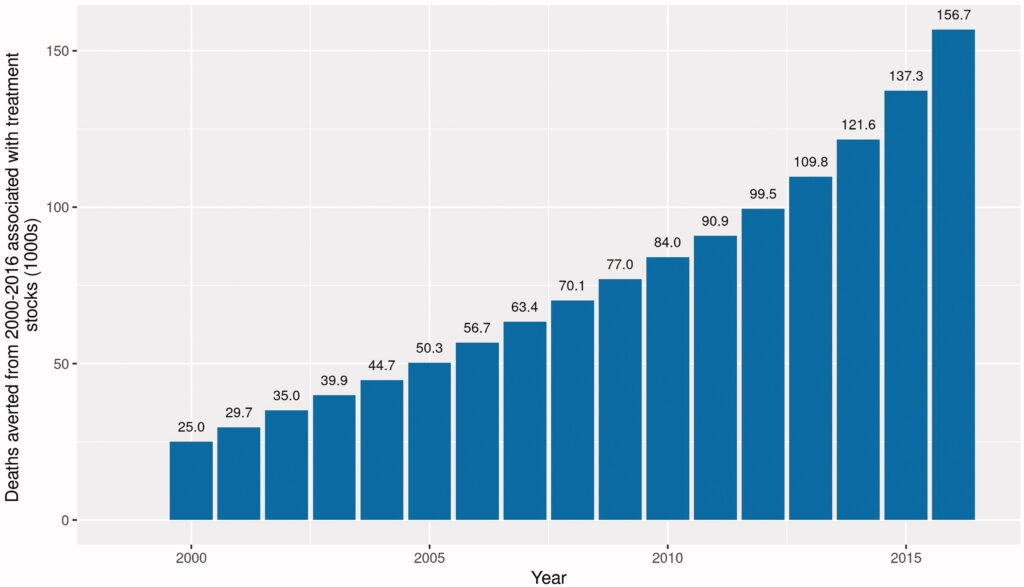
Based on these data, the share of states with <80% coverage of Medicare coinsurance and deductibles has grown over time, from 24 states in 2004 to 29 states in 2018. Further, the number of states that provide full reimbursement fell from 11 in 2004 to 7 in 2018.
One limitation of the data used for this evaluation is that it ignores managed care. Medicaid managed care organizations (MCOs) and MCOs are have grown–relative to Medicaid fee-for-service–over time.
Lesser-of policies function similarly under Medicaid managed care, except that Medicaid MCOs pay the lesser-of (a) the difference between their negotiated provider rates and Medicare’s payment amount, and (b) Medicare’s cost sharing. However, to the extent payment rates negotiated by Medicaid MCOs differ from those in fee-for-service Medicaid, our payment index will not accurately reflect provider payments for duals enrolled in Medicaid MCOs.
Further, the analysis also does not include Medicare Advantage beneficiaries. In 2018, Medicare Advantage plans covered 33% of dual-eligibles with full Medicaid.
Nevertheless, the creation of this dataset to track changes in Medicaid dual-eligible generosity is certainly a useful contribution to the literature.
Source:
from Healthcare Economist https://ift.tt/3285iAA
via
IFTTT